Bone-Anchored Prosthesis
The research study will involve:
Nationwide enrollment in a Clinical Study of the Transdermal Compress bone-anchored prosthetic implant system.
Clinical Study participants are committing to five years of study participation.
Participants will be asked to complete questionnaire regarding prosthetic use and overall health using a study app on their phone.
General Participation Criteria:
Age: 18 – 60
Weight: ≤ 245
Transfemoral amputee
No nicotine use

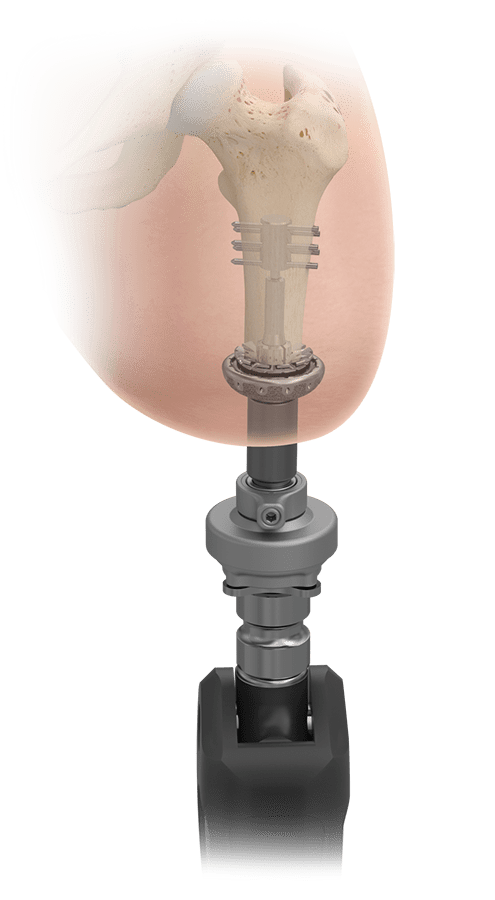
What is Transdermal Compress?
The Transdermal Compress investigational device is a bone anchored implant that allows for direct attachment of a prosthetic leg without the use of a socket. It is intended for above-the-knee amputees who experience problems with, or cannot use, a conventional socket prostheses.
Study Locations
No matter your location throughout the US, you can contact any of the participating institutions listed below for more information regarding eligibility.
California
UC Davis Medical Center
Recruiting
Sacramento, California
Contact: Marisa Arnold
Colorado
University of Colorado Anschutz Medical Campus
Recruiting
Aurora, Colorado
Contact: Eric Early
District of Columbia
Walter Reed Military Medical Center
Recruiting
Washington, D.C.
Contact: Emma Stewart
Illinois
Northwestern Memorial Hospital
Recruiting
Chicago, Illinois
Contact: Melissa Shauver
Maryland
The Johns Hopkins Hospital
Recruiting
Baltimore, Maryland
Contact: Malcolm Hamilton-Hall
New York
Memorial Sloan Kettering Cancer Center
Recruiting
New York, New York
Contact: Serena Sim
Ohio
The Ohio State University Wexner Medical Center
Recruiting
Columbus, Ohio
Contact: Emily Rice
Pennsylvania
Penn Medicine; University of Pennsylvania Health System
Recruiting
Philadelphia, Pennsylvania
Contact: Warren Harding
Pennsylvania
University of Pittsburgh Medical Center
Recruiting
Pittsburgh, Pennsylvania
Contact: R McGough, MD
412-802-4100
Contact: B Krawczyk
412-605-3003
